Two papers focusing on the ribosomes were recently published. The first paper highlights the discovery of an area of twofold pseudosymmetry (SymR) within the peptidyl transferase center of the modern ribosome (Rivas and Fox 2020). This observation strongly suggests that the very core of the ribosome arose from a dimerization event between two modest-sized RNAs. It was previously shown that at least four non-standard interactions exist between the two halves of SymR. In this new paper, the structure of the SymR has been verified to be highly conserved with respect to both ribosome transition state and phylogenetic diversity. These comparisons also reveal two additional sites of interaction between the two halves of SymR and refine our understanding of the previously known interactions. In addition, the possible role that magnesium may have had in the coordination, stabilization, association, and evolutionary history of the two halves (A-region and P-region) was discussed. Together, these results identify a likely site where structural elements and Mg2+ ions may have facilitated the ligation of two aboriginal RNAs into a single unit. Furthermore, metallic cations like magnesium might be relevant not only for the folding and catalytic capabilities of the proto-ribosome, but they also play a role in the evolutionary pathway this structure might have followed.
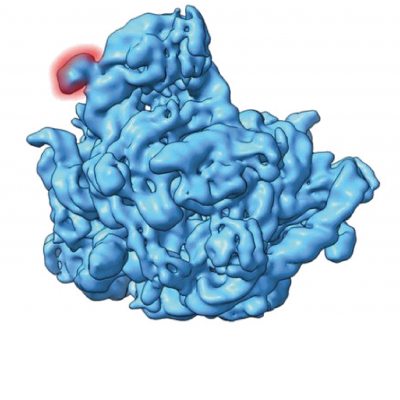
The second paper highlights the cryo-electron microscopy (cryoEM) structural validation of an insert (expansion segment) in the 5S ribosomal RNA sequence of Halococcus morrhuae (extreme salt loving / requiring microbes belonging to Archaea) (Tirumalai et al. 2020) This 108‐nucleotide insertion which almost doubles the length of the 5S rRNA was first identified in 1981 (Luehrsen et al. 1981). The current published work validates the insert using cryo‐electron microscopy reconstruction of the native large subunit at subnanometer resolution of 6.4 angstroms. Not only is the insert clearly visible as a well-defined blob on the cryoEM image, the insert site forms a four‐way junction that fully preserves the canonical 5S rRNA structure. The inserted region is conformationally flexible and does not pack tightly against the large subunit. The high‐salt requirement of the H. morrhuae ribosomes for their stability conflicted with the low‐salt threshold for cryoEM procedures. Despite this obstacle, this is the first cryo‐electron microscopy map of Halococcus ribosomes.
Understanding the evolution of the ancient macromolecules, namely the ribosomes, is central to understand how biology evolved. While the ribosome core is very conserved across all of biology, as exemplified by the first paper (Rivas and Fox 2020), the differences are in the form of these extensions (or deletions in some cases), such as the 5S insertion reported in the second paper (Tirumalai et al. 2020). In the light of insertions and extension sequences, the addition of ribosomal segments goes in parallel with the increase in ribosomal complexity. Thus, structural elucidation of such ribosomal expansion segments is vital towards understanding the same.
References
