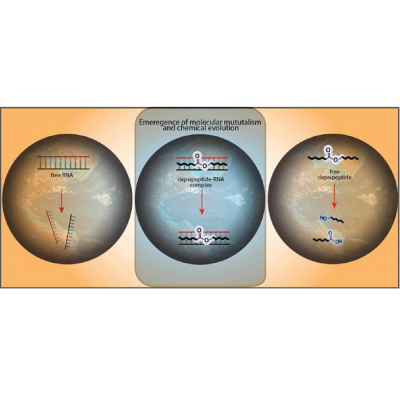
Cooperative relationships between different classes of biopolymers in today’s biochemistry, such as peptides and nucleic acids, is suggestive of a functional co-evolution between early proto-polymers. Here we show that cationic protopeptides (depsipeptides and polyesters), either produced as mixtures from prebiotic dry-down reactions or synthetically prepared, can engage in direct interactions with RNA resulting in mutual stabilization. Specifically, cationic protopeptides increase the thermal stability of folded RNA, and RNA increases the lifetimes of proto-peptides. It is generally believed that proteinaceous cationic amino acids (Lys, Arg, and His) were not abundant on prebiotic Earth while amino acids with shorter cationic side chains, such as Orn, Dab, and Dpr, are thought to have been more abundant. Proteinaceous amino acids adjacent to backbone ester bonds generally promote greater RNA duplex thermal stability compared to analogous sequences containing non-proteinaceous residues. The side chains of Lys, Arg, and His have a lower likelihood of undergoing intramolecular reactions, thus reducing the chances of chain termination and product degradation. Our findings support a model in which biological dependencies of RNA and protein reflect a long co-evolutionary history that began with mutually stabilizing interactions at early stages of polypeptide and nucleic acid co-existence.
For more information, check out Frenkel-Pinter’s blog post on Nature Research Chemistry Community.
Frenkel-Pinter, M., Haynes, J.W., Mohyeldin, A.M. et al. Mutually stabilizing interactions between proto-peptides and RNA. Nat Commun 11, 3137 (2020). https://doi.org/10.1038/s41467-020-16891-5
