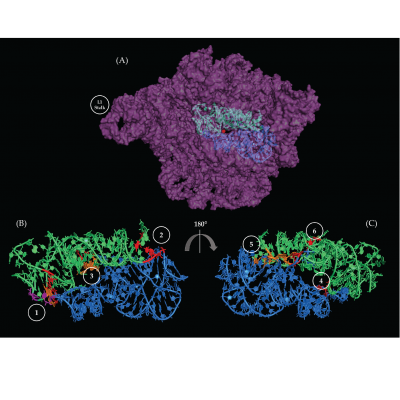
Peptide bond synthesis occurs in the peptidyl transferase center (PTC) of the large ribosomal subunit (LSU). There is an area of twofold pseudo-symmetry (SymR) in and around the PTC comprised of about 178 nucleotides. It has two halves, one half associated with the ribosomal A site, and the other with the P site. SymR is theorized to have emerged by an heterodimerization event in the prebiotic world, likely utilizing RNA/RNA interactions to form a stable structure. Previous studies have stated that the two halves were stabilized by four non-standard interactions. Rivas and Fox used the LSU crystallographic structures, from which the SymR was dissected, that represent four different transition states to verify, describe, and analyze the nature of up to a total of six regions of RNA/RNA interactions. Moreover, SymR structures derived from several phylogenetically distant organisms were examined and results strongly suggest that the A- to P-region stabilizing interactions are phylogenetically conserved and are upheld across ribosomal transition states. The conservation of these six interaction regions suggests a role in the stability of the PTC and possible contribution to the assembly of the so called “proto-ribosome”. The role of magnesium in the folding and evolution of SymR was also studied. 28 magnesium ion contacts were observed, but only 2 established contacts between the A- and P-regions of the SymR. The Mg2+ ions were classified into three categories to try and give insight about the relative time in which these interactions could have been established. These magnesium ions exhibit different degrees of conservation over crystallographic structures from organisms across the three domains of life. Nevertheless, the results suggest that metallic cations may be relevant for the folding, evolution, and catalytic capabilities of the SymR and the emergence of the “proto-ribosome”.
Rivas M, Fox GE. Further Characterization of the Pseudo-Symmetrical Ribosomal Region. Life. 2020; 10(9):201. https://doi.org/10.3390/life10090201
