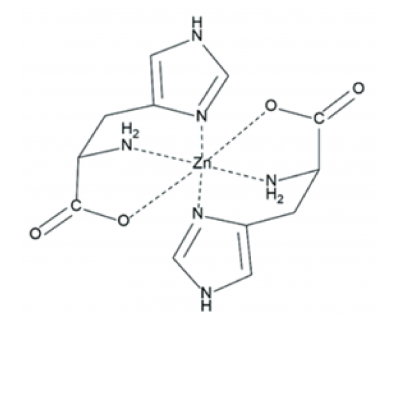
The authors tested the effect of Zn2+ and other metal ions on polymerization of histidine (His) and glycolic acid into depsipeptides in dry-down reactions. The results here show that interactions of depsipeptides with metal ions could have influenced early chemical evolution of proto-peptides. Data showed that addition of Zn2+ increased the average lengths of His-containing depsipeptides. This is likely caused by direct association between His monomers and Zn2+ since the effect is dose dependent and direct binding is observed via circular dichroism. To determine if oligomerization of additional imidazole-containing monomers besides His is enhanced by Zn2+, L-β-imidazole lactic acid was dried with glycolic acid to form polyesters. Addition of Zn2+ increased the yield of longer polyester oligomers but did not change the extent of conversion of monomers to oligomers. These results imply that a terminal alcohol can also support a chelation complex with Zn2+. To determine if the effect of Zn2+ on depsipeptide formation is specific to His, Zn2+ was added to dry-down reactions involving alanine or lysine in the presence of glycolic acid . It was found that Zn2+ inhibited depsipeptide formation for both, demonstrating that the effect of Zn2+ on depsipeptide formation is not a generic effect and is specific to His. Other metals were tested and it was found that Cu2+ and Co2+ increased the production of His-containing depsipeptides, while Na+, K+, and Mg2+ did not. On the other hand, Ca2+ decreased the production of depsipeptides. Circular dichroism analysis showed inversion of the spectrum upon addition of Zn2+ which may result from formation of His- Zn2+ complex in concert with a change in His conformation. The inversion was observed for Co2+, Cu2+, or Ni2+, but not for the alkali or alkali earth metals. This indicates that the low-lying d orbitals of metals are important since the valence electrons tend to promote stable coordination complexes. The plausible importance of small proto-metalloproteins on the prebiotic Earth is supported by the vast cooperative interactions of metals and proteins in extant biology. If accumulation of metals occurred at shallow lakes or similar environments that were subjected to dry-wet cycling, they might have affected distribution of polymers that formed within these environments in a specific manner.
Frenkel-Pinter, Moran & Sargon, Alyssa & Glass, Jennifer & Hud, Nicholas & Williams, Loren. (2021). Transition metals enhance prebiotic depsipeptide oligomerization reactions involving histidine. RSC Advances. 11. 3534-3538. 10.1039/D0RA07965K
